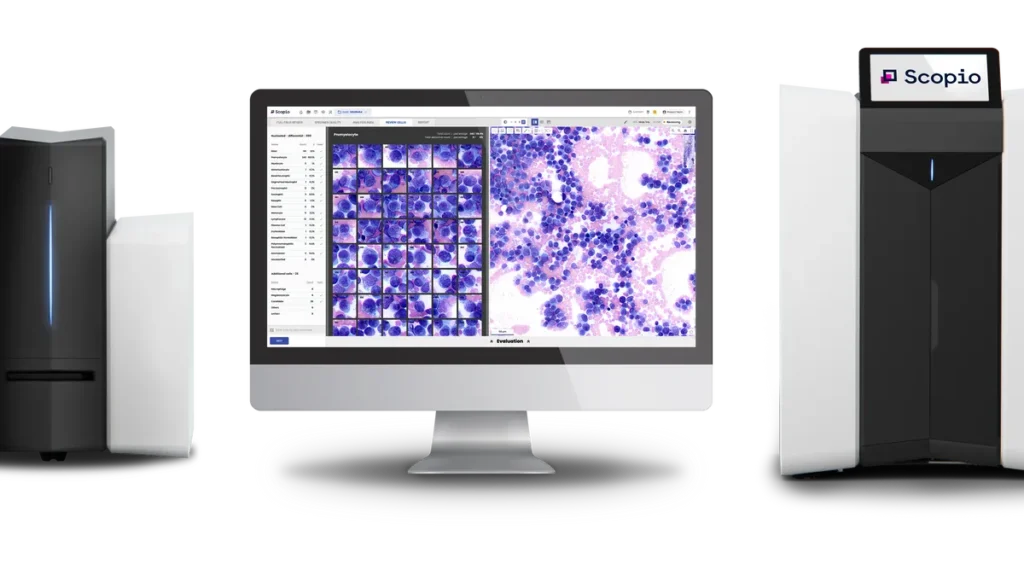
Scopio Labs has received de novo authorization for a device that finds and presents images of cells in bone marrow smears.
The Food and Drug Administration authorization clears Scopio to add capabilities to its X100 and X100HT devices, imaging platforms that allow users to view blood samples digitally rather than under a microscope. Scopio announced the de novo clearance on Wednesday.
Siemens Healthineers struck a deal to distribute Scopio’s platforms in 2023. The company identified the technology as a way to make laboratory workflows more efficient..