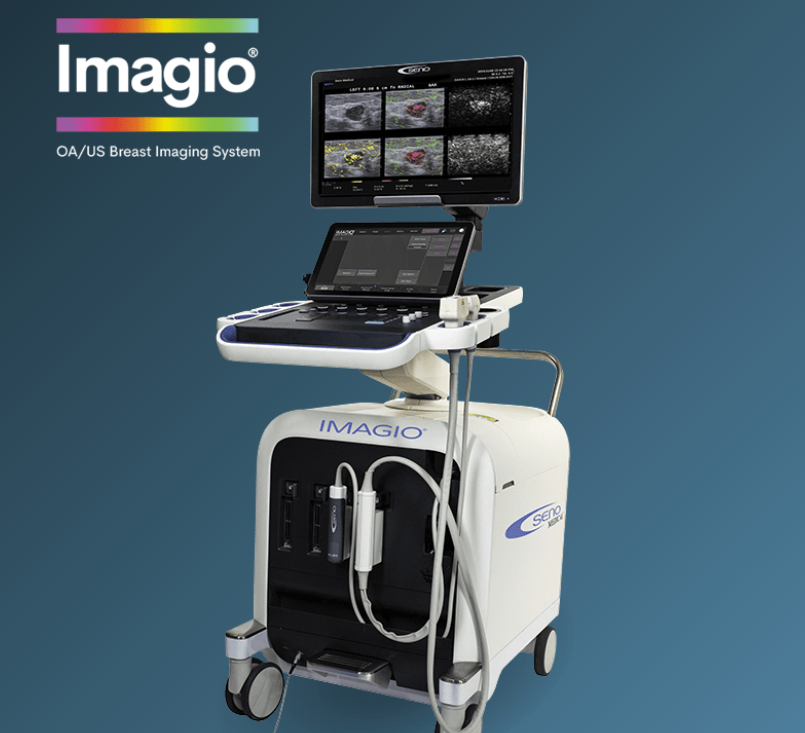
SAN ANTONIO, Jan. 28, 2026 /PRNewswire/ — Seno Medical has received CE Mark certification for its next-generation Imagio® Imaging System, Model 9100. EU MDR CE Mark indicates that a medical device meets the stringent safety, performance, and quality standards established globally, assessed by notified bodies approved for granting certification in the EU, and is mandatory for sale of product in the European Union and beyond.
“EU MDR certification is one of the most challenging regulatory processes, and we are so pleased to have achieved this significant milestone for our most recent version of the Imagio® System,” commented Tom Umbel, CEO of Seno Medical. “Imagio® delivers a revolutionary leap forward in patient care, and we are thrilled to be able to collaborate with our European colleagues to improve diagnostic processes for providers and patients.”