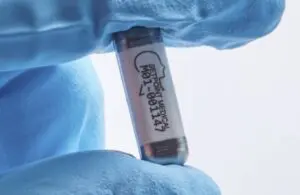
Valencia, California–based SetPoint designed its nerve modulation technology for people with relapsing-remitting multiple sclerosis (RRMS). Breakthrough designation enables more communication and priority regulatory review with the FDA. Plus, it supports reimbursement and patient access upon approval.
This isn’t the first breakthrough nod for SetPoint’s technology. It picked up the designation in 2020 to treat rheumatoid arthritis (RA). The company continues evaluating the investigational platform in a pivotal clinical trial for the treatment of RA.