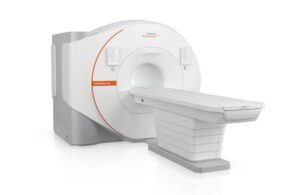
The 1.5T platform features a closed helium circuit and no quench pipe, which reduces helium dependency and annual energy consumption. Siemens Healthineers said this aims to reduce helium dependency and annual energy consumption. The upgradeable platform covers the entire range of MR applications.
Magnetom Flow offers image reconstruction based on AI for shorter scan times with improved image quality. It marks the second virtually helium-free 1.5T MR platform from Siemens Healthineers, following the Magnetom Flow Ace.