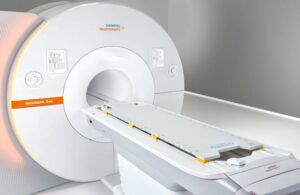
The PET/MR (positron emission tomography/magnetic resonance) scanner enables simultaneous visualization of organ location, function and cellular metabolism. The company says PET/MR can potentially reveal a tumor’s location and its metabolic activity and response to treatment. With these capabilities, Biograph One can deliver enhancements in theranostics for personalized oncology treatments.
Siemens Healthineers said in a news release that studies also suggest that PET/MR could become a tool for neurodegenerative diseases. The company said emerging drug therapies for diseases like Alzheimer’s rely on PET scans to confirm a diagnosis, while MR scans monitor related side effects.