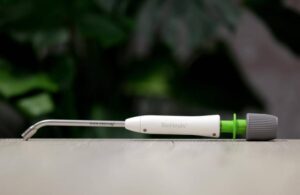
The company describes BioHealx as a first-of-its-kind medical device that offers a single-use, bioabsorbable option to treat anal fistula in a minimally invasive procedure. Galway, Ireland–based Signum designed it to close the internal opening of the fistula tract via tissue apposition. It then dissolves in the body after treatment.
Signum said it designed the single-operation approach to promote healing, prevent fistula recurrence and protect patient continence.
De novo clearance follows the completion of a single-arm, non-randomized clinical trial in 2023. The trial included 32 participants who experienced recurrent anal fistula from at least one previous failed treatment. It also includes follow-up from those patients over a period ranging from 13-40 months.