FDA clearance came just days after the company inked a partnership with Mayo Clinic to develop precision medicine solutions.
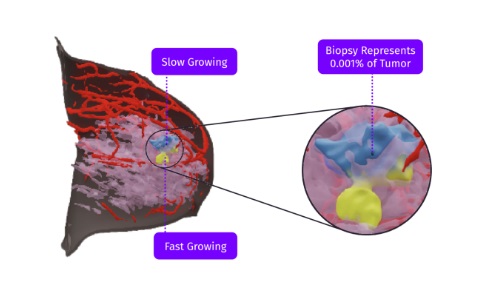
The TumorSight platform takes a patient’s standard-of-care imaging to build a custom 3D model of their tumor. The tool provides 3D spatial visualizations of breast cancer to support more effective planning and consultations. SimBioSys said it gives clinicians and patients a more comprehensive understanding of their cancer and the potential options available.
Additionally, the TumorSight application provides insights like tumor volume, tumor-to-breast volume and tumor distance to key anatomical structures. It quantifies key metrics required in treatment planning.