“Innovative, patient-centric technologies like Archimedes™ that simplify care, reduce patient burden, and enhance the home dialysis experience have the potential to transform kidney care and enable more patients to successfully adopt home therapies. Congratulations to the Simergent team on receiving FDA 510(k) clearance for the Archimedes™ automated peritoneal dialysis cycler,” stated Dr. Jeffrey Perl, expert nephrologist in peritoneal dialysis care and Editor-in-Chief of the Peritoneal Dialysis International journal.
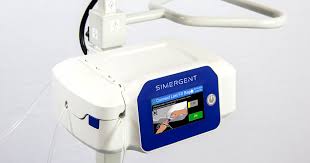
Archimedes™ is a home dialysis system with a user-friendly interface designed for patient and clinic users with a wide variety of educational and socioeconomic backgrounds. Its 4-hour battery life and integrated mobile cart allows unparalleled in-home mobility while performing dialysis. The patented fluid delivery and measurement technology requires no pump, which supports quiet operation for this nocturnal therapy. Archimedes™ incorporates a patented shroud to support aseptic tubing/bag connections. Its fast 5-minute therapy setup time allows patients to spend less time waiting for the system to perform pre-therapy checks.