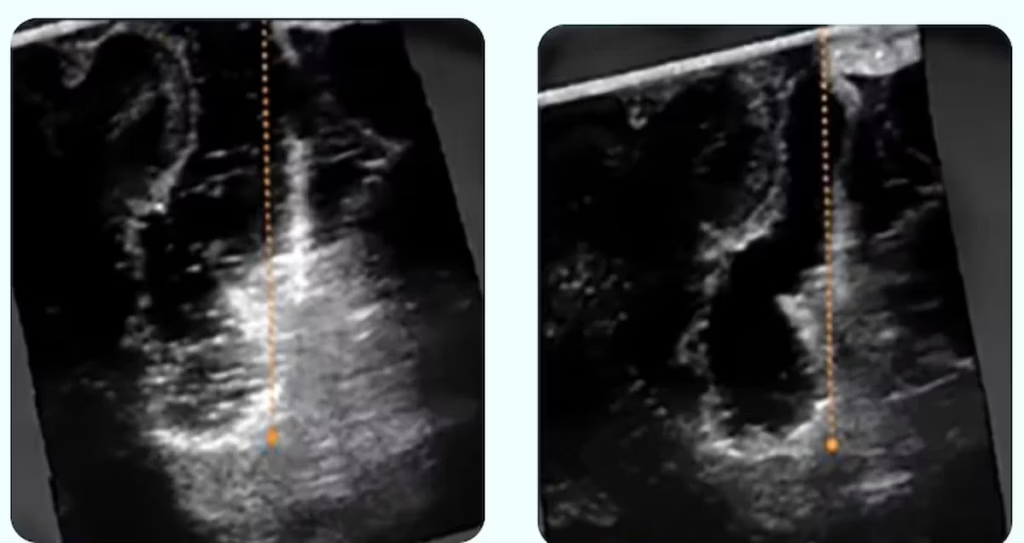
The SonoClear System is an acoustic coupling fluid and sterile transfer kit, engineered to address a critical limitation of conventional intraoperative imaging during neurosurgical interventions. The SonoClear System’s tissue-mimicking properties remove the acoustic artifacts that appear when using standard irrigation fluids as a couplant and which typically obscure critical tumor remnants at the base of a resection cavity. The SonoClear System was developed, and is wholly owned, by SonoClear, a company founded to improve diagnostic accuracy in ultrasound-guided interventions.
“Removing aggressive neurological tumors, such as gliomas, presents a significant surgical challenge,” said Prof. Geirmund Unsgaard, neurosurgeon and SonoClear founder. “In brain tumor surgery, neurosurgeons rely on intraoperative ultrasound to guide their work and confirm complete tumor removal. Standard irrigation fluids create visual artifacts that can obscure the surgical site precisely when clarity is most critical: at the end of the procedure when surgeons need to verify whether any tumor remains. We created a simple solution that works with all intraoperative ultrasound systems and allows neurosurgeons to clearly see the tumor, enabling improved decision-making in surgery.”