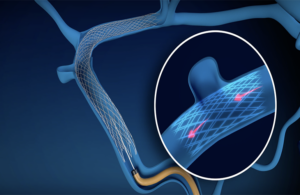
“The BosSTENT represents years of focused innovation to deliver an on-label, minimally invasive solution that normalizes venous hemodynamics and has the potential to dramatically improve quality of life for individuals with pulsatile tinnitus.” Sonorous Neurovascular President Joel Harris said.
BosSTENT is a novel braided, self-expanding stent that differs from traditional mesh stents; and features optimal radiopacity, enhanced visibility and resheathability, according to the Lake Forest, California-based company.