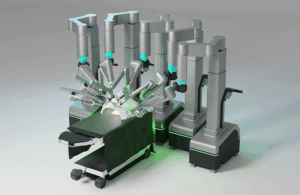
The company — which has locations in Gurugram, Haryana, India and Fort Lauderdale, Florida — said it submitted the robot on Dec. 5, 2025, seeking a range of indications. Those include general, urological, colorectal, gynecological and cardiac surgery. It opted for the 510(k) submission rather than a de novo request based on a pre-submission meeting and subsequent discussions with the FDA.
Mantra features a modular, 3D vision, open-console design with ergonomics that the company describes as superior. It engages machine learning models to improve safety and efficiency during procedures.
SS Innovations previo