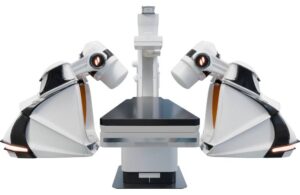
GenesisX marks the latest advancement in endovascular surgical robotics, the St. Louis-based company says. It builds on the company’s established robotic magnetic navigation (RMN) technology while enhancing its accessibility.
Clearance for the endovascular surgical robot comes just days after Microbot Medical launched its own endovascular robot, which won FDA clearance in September. Other potential entrants into the endovascular robot space with recent milestones include Remedy Robotics and Sentante.
More on Medical Design & Outsourcing: Why Stereotaxis says magnets are so attractive for minimally invasive medtech