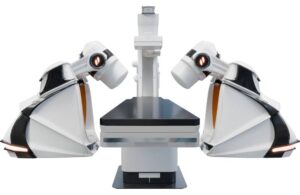
The GenesisX system is the latest iteration of Stereotaxis’ Genesis robotic magnetic navigation (RMN) system. It builds upon the company’s technology while reducing the complexities and barriers to hospital adoption.
Stereotaxis said in a news release that RMN systems require significant preparations within an operating room. That includes structural modifications, installing thousands of pounds of magnetic shielding, floor reinforcement, high electrical power and extensive cabling.
To address this, GenesisX utilizes smaller magnets and incorporates magnetic shielding into its structure. It requires no structural anchoring through the floor and uses standard 120/230V power outlets. A single fiber connects each robot to the system cabinet, which is 80% smaller than the previous generation of Genesis. The system can fit under a table in an operating room and maintains the speed, responsiveness and workflow of Genesis. The company plans to use it as a platform for additional innovations in the future.