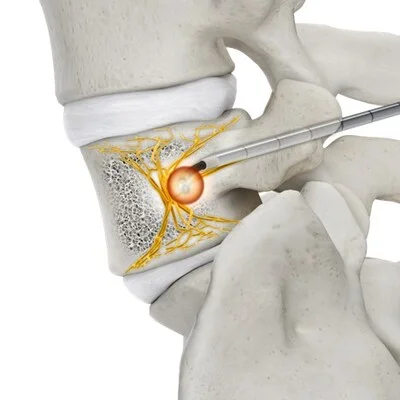
Stryker’s OptaBlate® Basivertebral Nerve Ablation (BVN) System, recently granted 510(k) clearance by the U.S. Food and Drug Administration (FDA), marks a major advancement in the treatment of chronic vertebrogenic low back pain. Designed with precision-focused radiofrequency ablation technology, the system includes a steerable curved introducer, microinfusion to reduce charring and impedance errors, and a fast lesioning capability that creates a 1 cm ablation in just seven minutes. These innovations streamline the procedure and enhance safety, enabling physicians to deliver consistent, controlled results with minimal tissue disruption.
This minimally invasive system is particularly significant for patients with chronic low back pain linked to Modic type 1 or 2 changes—an often underdiagnosed and undertreated subset. Traditional conservative therapies like physical therapy, injections, or medication frequently fall short of providing lasting relief. OptaBlate BVN directly targets the basivertebral nerve, which transmits pain signals from damaged vertebral endplates. By ablating this nerve, the system offers a durable, non-surgical solution that can significantly improve patient outcomes and potentially delay or eliminate the need for more invasive interventions.