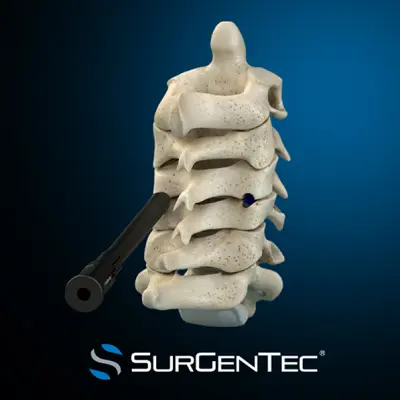
SurGenTec’s ION-C facet fixation system, an implantable screw used to stabilise the posterior cervical spine’s (neck) facet joints during spinal fusion procedures, has received an expanded indication from the US Food and Drug Administration (FDA) for use in cervical pseudoarthrosis treatment.
Cervical pseudoarthrosis occurs when a previous neck fusion fails to heal properly. Inadequate surgical technique and fixture, or preexisting patient conditions such as osteoporosis, poor nutrition, or a history of smoking that results in restricted blood flow in the neck region, can make the failure of a neck fusion more likely.
The Florida-based company’s indication expansion for ION-C means that it can be used in corrective or revision surgeries to stabilise the neck’s facet joints, to maintain alignment and improve a surgery’s chances of success.