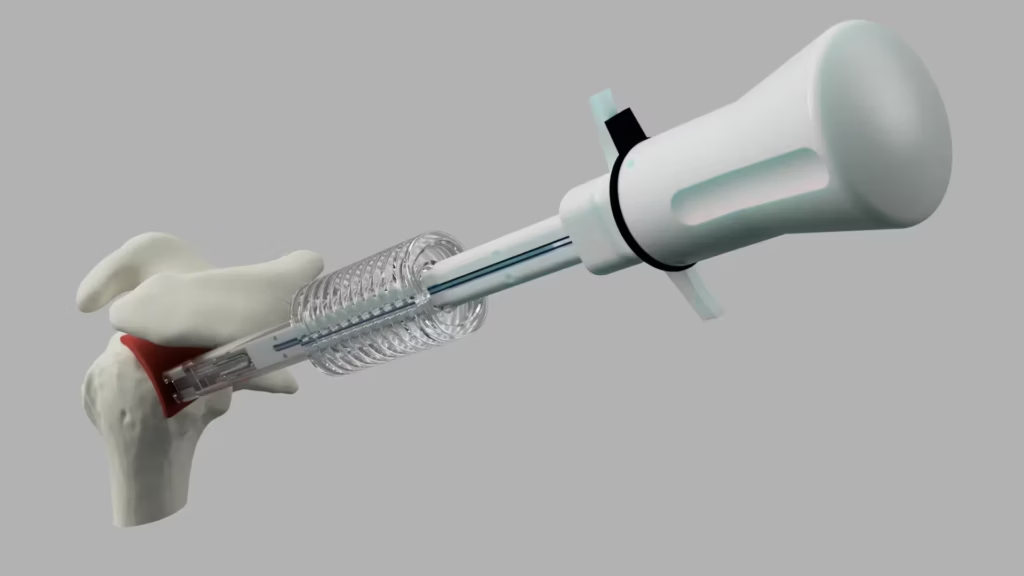
This clearance marks a major milestone in SutureTech’s mission to deliver clinically informed, surgeon-driven solutions that simplify complex procedures and expand market opportunities in musculoskeletal repair.
“Achieving FDA clearance for RapidFix™ is a defining moment for SutureTech,” said Dr. Oke Anakwenze, Founder & CEO of SutureTech and Chief of Shoulder Surgery at Duke University. “The device was designed to streamline surgical steps while maintaining strong, reliable fixation. We believe RapidFix™ offers value for surgeons seeking reproducibility—and for patients who benefit from more consistent repairs.”
Designed by Surgeons, for Surgeons
RapidFix™ is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs while reducing procedural steps and surgical complexity. Developed through eight years of surgeon-led collaboration, the device has been validated through preclinical testing for safety and performance.