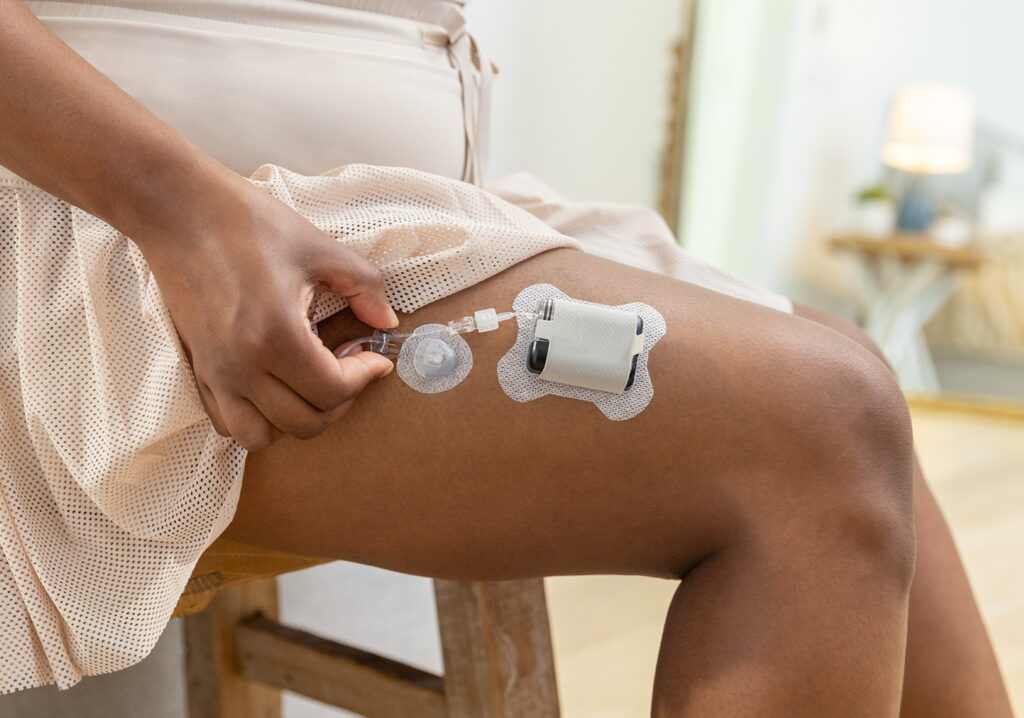
Our sister site, Drug Delivery Business News, reports today that the company shared in an SEC filing that its Capillary Biomedical (CapBio) subsidiary won FDA 510(k) clearance for a new infusion set.
The company won clearance for its SteadiSet Infusion Set, according to an SEC Form 8-K. It’s a wearable infusion set that delivers insulin from an insulin pump to the body. San Diego-based Tandem itself develops multiple automated insulin delivery systems, including the flagship t:slim X2 and Mobi miniature durable pump. The company could also eventually bring a patch pump system to market.
CapBio’s infusion set technology came to Tandem Diabetes Care through a 2022 acquisition for an undisclosed amount.