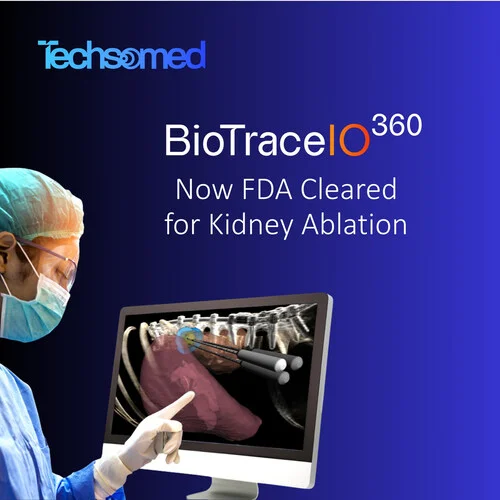
Techsomed, a leader in AI-Driven Precision for Image-Guided Care, announces U.S. FDA 510 (k) clearance expanding indications for BioTraceIO360* software platform to include percutaneous ablation of soft tissue in the kidney, in addition to its previously cleared liver application. The milestone advances Techsomed’s vision for a multi-organ, hardware-agnostic image-guided therapy (IGT) platform that standardizes minimally invasive care from planning through verification.