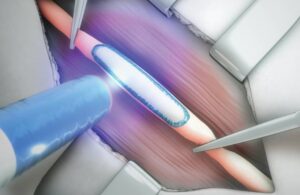
The Paris-based company, which has its U.S. headquarters in Cambridge, Massachusetts, designed Coaptium Connect as a first-of-its-kind atraumatic sutureless solution for peripheral nerve repair.
According to Tissium, the authorization further validates its biopolymer platform and enables U.S. commercialization of its first product. The company says the de novo nod makes Coaptium Connect the only FDA-authorized system designed for atraumatic sutureless nerve coaptation.