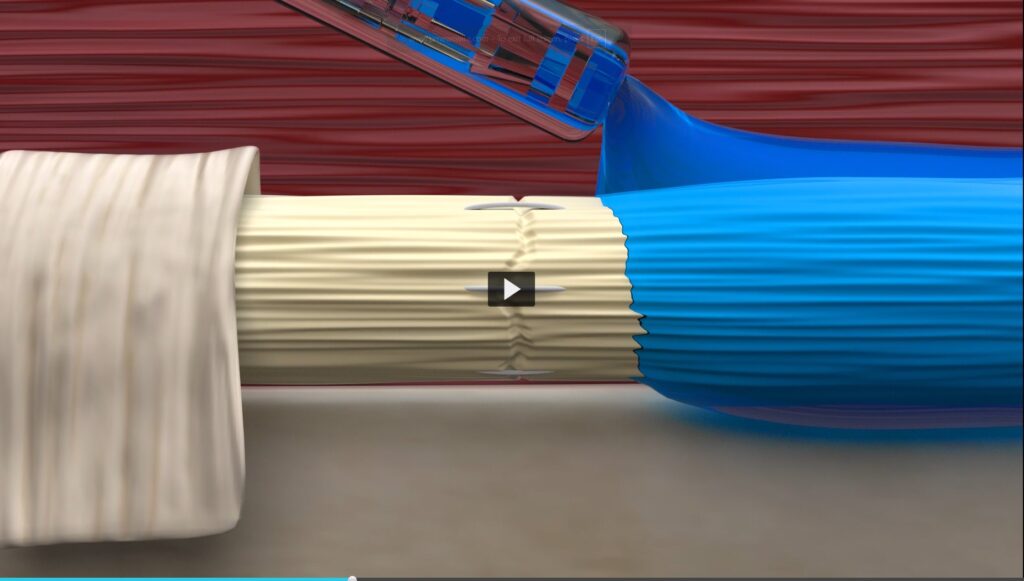
This clearance marks a significant milestone for TYBR Health, enabling a new approach to support functional recovery in musculoskeletal surgery. B3 GEL™ helps maintain surgical precision by temporarily separating tissues during the early healing phase—without impeding the natural healing process.
“When we started TYBR, it was with a clear purpose: to solve a real, overlooked problem in surgical recovery,” said Tim Keane, PhD, Cofounder and CEO of TYBR Health. “Too often, healing goes unprotected once the procedure ends. B3 GEL™ is designed to fill that gap—working with the body’s biology to protect tissue planes and give surgeons a way to safeguard patient outcomes during the critical early phase of recovery.”
B3 GEL™ is composed of a naturally derived extracellular matrix and is applied via TYBR’s proprietary integrated mixer-applicator system. Its flowable format conforms to complex anatomy and can be used in both open and minimally invasive procedures. The blue-colored gel allows for visual confirmation of placement and resorbs completely over time without leaving residual bulk.