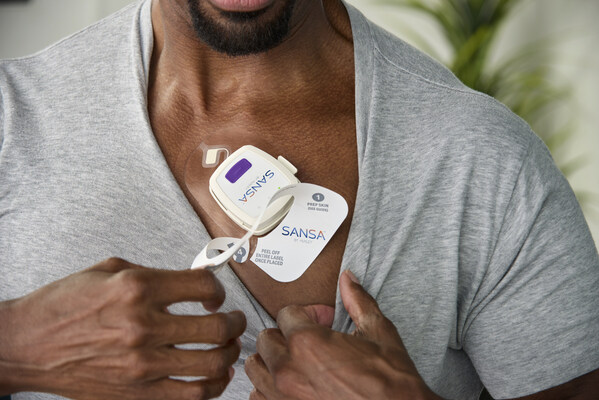
The patch is a first-of-its-kind, at-home, single-point-of-skin-contact diagnostic device and represents a breakthrough in medical innovation. Unlike current patches, rings, watches, or finger probes, SANSA does not require additional attachments, wires, belts, or hoses.
Its patented combination of sensors and materials precisely measures eight physiological channels including blood oxygen saturation, EKG-derived heart rate, respiratory effort, chest movement, sleep staging, snoring, body position, and actigraphy.