“Our goal is to allow the staff’s focus to remain on the patient,” commented Edgar Alvarez, Vice President of the X-ray portfolio in the U.S. “We hone in on that by giving them an intuitive user experience, streamlined workflows, and fantastic image quality at low dose.”
Specifically, the uAngio AVIVA offers the following:
Focus on what Matters – Intelligent Voice Assist and 3D Vision
An industry first, the intelligent system enables hands free image review and movement assistance allowing clinical staff the ability to focus on the patient during procedures in the interventional suite.
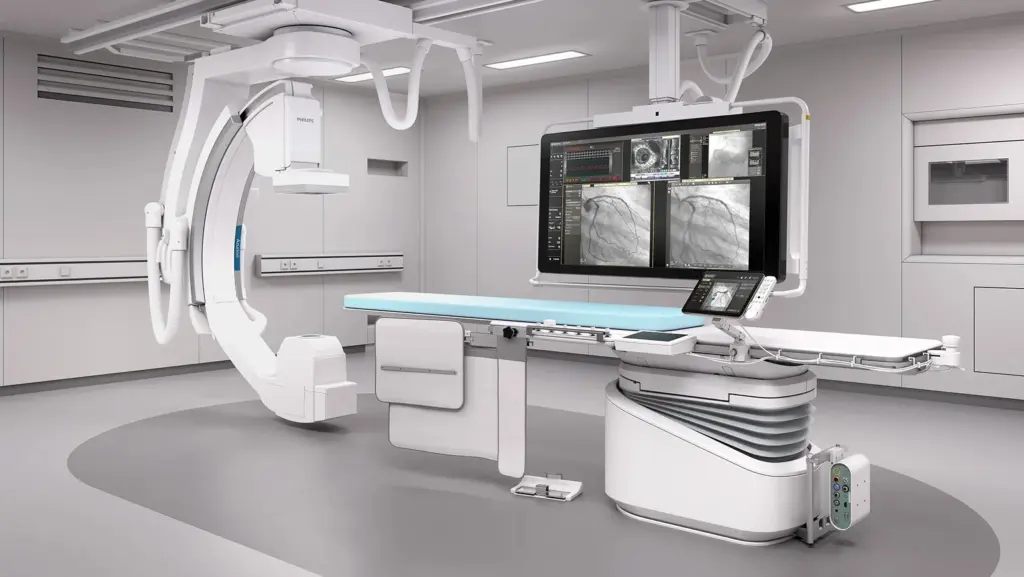
Clinical Flexibility Redefined – 8-Axis Robotics
The 8-axis ceiling-mounted robotic system offers exceptional flexibility, allowing full lab coverage, easy tableside access while navigating the complexity of medical equipment in the interventional site.
Imaging Insights Without Compromise
The uVERA IQ intelligent imaging technology leverages United Imaging’s proprietary AI algorithms to obtain high-quality images at low dose for interventional radiology, neuro interventions, and interventional cardiology procedures.