HUNTINGTON BEACH, Calif. & GOLD COAST, Australia & HOUSTON–(BUSINESS WIRE)– BiVACOR, Inc., a clinical-stage medical device company developing a total artificial heart (TAH), today announced the United States Food and Drug Administration (FDA) has provided approval for The BiVACOR Total Artificial Heart (BTAH) to commence an investigational device exemption (IDE) for the first-in-human Early Feasibility Study (EFS).
The EFS will evaluate the safety and feasibility of BTAH as a bridge to a heart transplant in the treatment of subjects with biventricular heart failure. The EFS has ten hospital location options and will initially enroll three patients, one leading hospital being the Texas Heart Institute in Houston, TX. The study is anticipated to commence in 2024 and will pave the way for a subsequent pivotal study.
“I am eager to begin the BiVACOR Total Artificial Heart EFS to evaluate what I believe is a promising and potentially life-saving technology,” said Joseph Rogers, MD, National P.I. and CEO of the Texas Heart Institute. “The implantation of a TAH system is a potential treatment option for patients with heart failure who need support while on the heart transplant waiting list and for those who do not qualify for a transplant. The BTAH is designed to replace the function of the native heart completely. It is an impressive technology, and I am excited to see the potential of BTAH in treating patients with severe heart failure.”
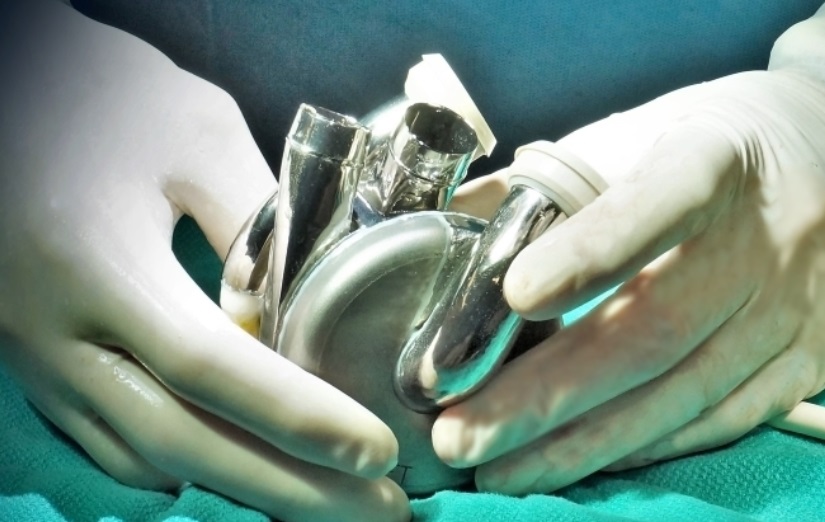
The BiVACOR Total Artificial Heart is designed as the first long-term therapy dedicated for patients with severe biventricular heart failure as an implantable TAH based on rotary blood pump technology. Similar in size to an adult fist, it is designed to be small enough to be implanted in many women and some children yet capable of providing enough cardiac output to an adult male undergoing exercise. Using magnetic levitation technology, the same principle used in high-speed trains, the design includes left and right vanes positioned on a common rotor to form the only moving part, a magnetically suspended double-sided centrifugal impeller. Even though there are no valves or flexing ventricle chambers, the device is designed to create pulsatile outflow by rapidly cycling the rotational speed of the impeller. The non-contact suspension provides large blood gaps, which is expected to minimize blood trauma and eliminate mechanical wear to offer a durable, reliable, and biocompatible heart replacement.