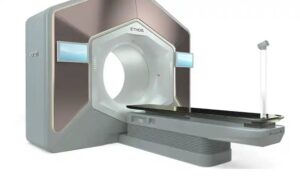
The company now introduces a streamlined workflow for daily treatment adaptation across more areas of the body. Ethos now also provides greater connectivity to patient data while reducing complexity, enabling adaptive, personalized radiotherapy.
Varian said Ethos 2.0 provides a versatile, comprehensive solution with a number of new and updated features. That includes enhanced algorithms for a more optimized approach to treatment planning and dose delivery, enabling faster treatment.
Images acquired using the HyperSight imaging solution on Ethos now integrate into the software to streamline workflows. This enables direct dose calculation for adapting treatments, helping clinicians create a treatment plan. Additionally, Ethos now significantly expands the number of AI-segmented anatomical structures that can be automatically contoured, expanding to more than 70 structures in the head and neck, thorax, abdomen, pelvis and bowel regions.