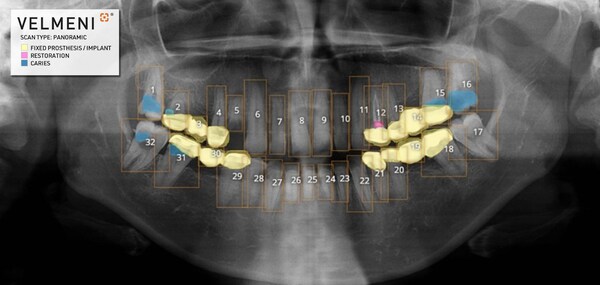
VELMENI for DENTISTS (V4D) assists dentists in the detection of dental caries, fillings/restorations, fixed prostheses, and implants in digital bitewing, periapical, and panoramic radiographs of permanent teeth in patients 15 years of age or older.
VELMENI is the first dental AI approved by the FDA to detect pathologies in panoramic X-Rays. VELMENI for DENTISTS adds its US FDA clearance to existing Canadian MDEL and New Zealand MEDSAFE clearances.
VELMENI for DENTISTS‘s AI-driven computer-aided clinical radiology solution is available to dentists throughout North America, Canada and many other parts of the world.
VELMENI collaborated closely with over 50 dentists, clinical members of VELMENI’s dental board, and 1300 dentists participating in a beta program that provided valuable and insightful feedback. Over 90% of dentists agreed VELMENI’s AI technology could be very helpful and said they would adopt it in their dental practice.