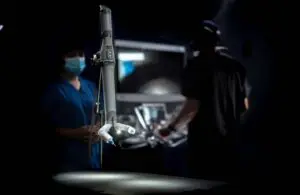
The FDA gave the nod for the use of the miniRAS (robotic-assisted surgery) device in adults undergoing colectomy procedures. MIRA went through the FDA’s de novo classification process, with findings based on an investigational device exemption (IDE) study.
Virtual Incision’s MIRA system features a small, self-contained surgical device. Inserted through a single midline umbilical incision in the patient’s abdomen, it allows for complex, multi-quadrant abdominal surgeries. The system also uses existing minimally invasive tools and techniques that are familiar to surgeons. Altogether, MIRA weighs about two pounds.
According to the company, the system’s tray-to-table design could provide the advantages of robotic surgery without requiring them to organize the operating room around the device — a current challenge with surgical robots