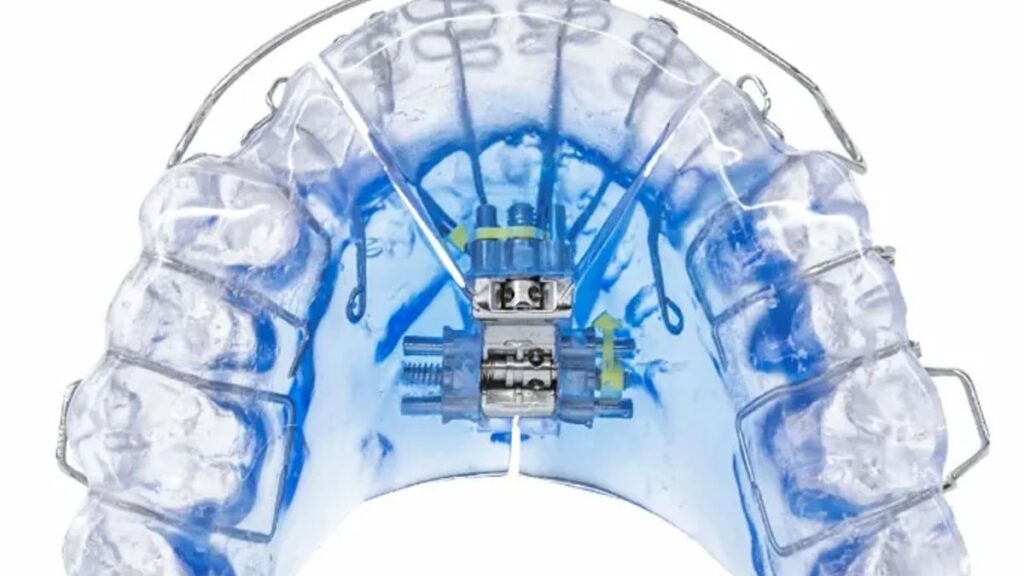
Vivos Therapeutics has received 510(k) clearance for the use of its oral devices in adults with severe obstructive sleep apnea (OSA).
The Food and Drug Administration ruling, which comes 11 months after Vivos received clearance for an oral appliance in mild-to-moderate OSA, gives the green light to an application that was previously only possible off-label. The company’s trio of devices, called Complete Airway Repositioning and/or Expansion (CARE) oral appliances, include the DNA, mRNA and mmRNA products.
Vivos’ share price increased nearly 834% to $41 when the market closed on Wednesday. The medtech company’s market capitalization was approximately $30.8 million even after the latest jump in its stock, according to Nasdaq.