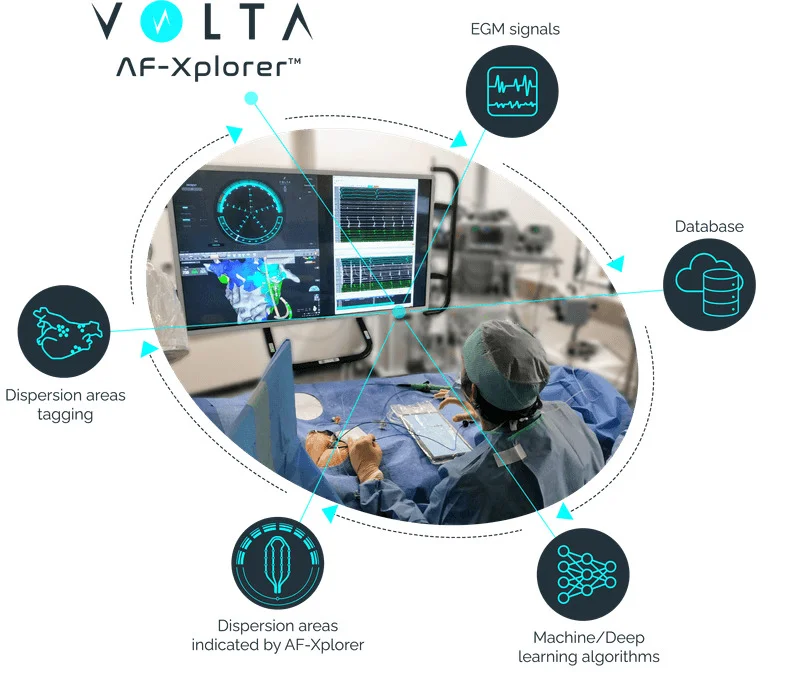
Volta Medical has reached a pivotal milestone with the U.S. label expansion of its flagship AI-driven system, Volta AF-Xplorer™, now incorporating clinical data from the landmark TAILORED-AF trial. The Volta AF-Xplorer delivers real-time, intelligent guidance to electrophysiologists during atrial fibrillation (AF) ablation procedures, enabling a personalized, data-informed approach to treating complex arrhythmias. With this update, the system becomes the first in electrophysiology to be supported by Level 1 clinical evidence demonstrating superior efficacy in the treatment of persistent AF—a particularly challenging and under-treated form of the disease.
The TAILORED-AF study, a prospective, multicenter, randomized controlled trial, compared standard pulmonary vein isolation (PVI) procedures to Volta’s AI-guided tailored ablation strategy. The results were groundbreaking: 88% of patients in the tailored ablation arm remained free from AF at 12 months, versus 70% in the standard-of-care group (p<0.0001). Published in Nature Medicine, these findings validate the ability of Volta AF-Xplorer to improve long-term patient outcomes and represent a major advancement in the treatment landscape for persistent AF, which affects a majority of AF patients but is often inadequately addressed through conventional methods.