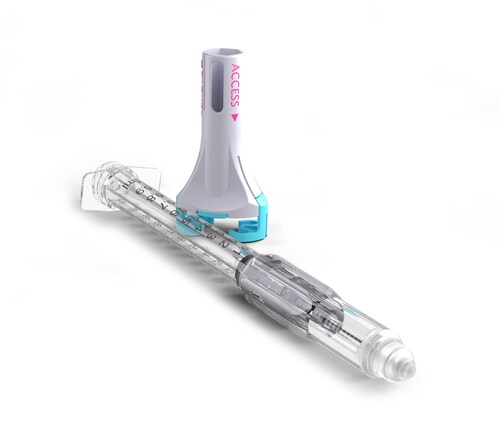
The VaporShield™ CSTD is the latest innovation in a portfolio of safety syringes being developed by Zephyrus. CSTDs are critical to protecting healthcare workers from hazardous drug exposure, which is now required in the US under USP<800> legislation. Currently, there are no options that meet this legislation for healthcare workers who deliver subcutaneous or intramuscular injections. VaporShield provides a true closed system from drug draw to injection and through to disposal, while retaining the crucial safety syringe features of its Aeroject™ auto-retractable syringe to prevent needlestick injuries. The global CSTD market is expected to reach around $2.7 Billion by 2030, with the US accounting for 40% of sales.