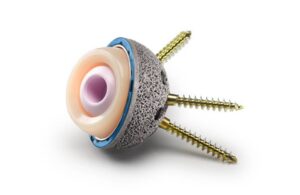
Warsaw, Indiana-based Zimmer Biomet designed the next-generation implant to address challenging primary and revision hip replacements. The company built it on its G7 platform, combining trabecular metal technology with an anatomic screw-hole pattern. It features Rim Fix screws and perimeter holes designed to provide multiple points of access and engagement to help surgeons achieve stable fixation in compromised bone.
According to Zimmer Biomet, the G7 shell’s trabecular metal porous surface is made from elemental tantalum. It has structural, functional and physiological properties similar to bone. The material features a 100% open, engineered and interconnected pore structure. This supports biological fixation and vascularization.