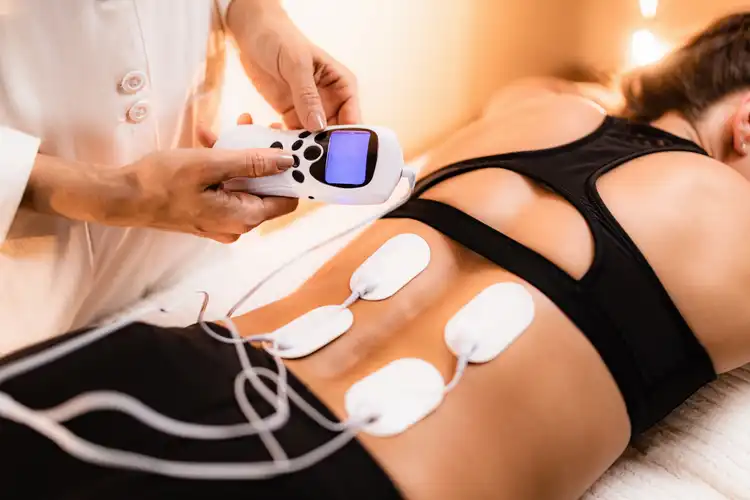
The TensWave device, by prescription only, builds on Zynex’s strong legacy of innovation in pain management. It offers a user-friendly, portable design that can be easily integrated into patients’ daily routines. The device aims to provide effective pain relief through TENS (Transcutaneous Electrical Nerve Stimulation) therapy, which has been clinically proven to reduce chronic and acute pain without needing medication.
“The introduction of TensWave aligns perfectly with our commitment to providing comprehensive pain management solutions,” said Thomas Sandgaard, CEO of Zynex Medical. “We recognized a gap in the market for a high-quality TENS device that meets the specific criteria for insurance reimbursement, and TensWave is our answer to that demand. It complements our flagship multi-modality device, the NexWave, where Interferential current is the main modality and driver of obtaining prescriptions. This device broadens our product portfolio and enhances our support to patients.”