FDA clears latest Philips TEE transducer for cardiac care
Philips (NYSE: PHG)+
announced today that the FDA granted 510(k) clearance to its latest TEE transducer technology.

Philips (NYSE: PHG)+
announced today that the FDA granted 510(k) clearance to its latest TEE transducer technology.

InspireMD (Nasdaq:NSPR) announced today that it received CE mark approval for its CGuard embolic prevention carotid stent system (EPS).
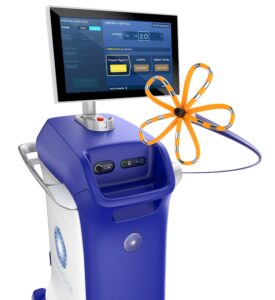
Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE™ Pulsed Field Ablation (PFA) System. The FARAPULSE PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., intermittent) atrial fibrillation (AF) and is a unique new alternative to standard-of-care thermal ablation treatment.

With improvements to the stent graft delivery system enabling a 1 Fr profile reduction on the majority of sizes, the device now offers the most 6 Fr compatible configurations among balloon expandable stent grafts.
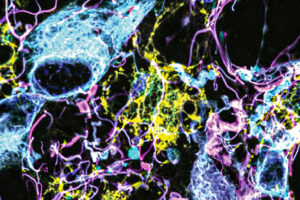
A new microscopy technique that enables high-resolution imaging could one day help doctors diagnose and treat brain tumors.