SetPoint Medical wins FDA breakthrough nod for nerve stim tech for multiple sclerosis
SetPoint Medical announced today that received FDA breakthrough device designation for its novel neuroimmune modulation platform.

SetPoint Medical announced today that received FDA breakthrough device designation for its novel neuroimmune modulation platform.

NEWPORT BEACH, Calif., March 13, 2024 /PRNewswire/ — Ventris Medical, a privately held orthobiologics and tissue regeneration company, today announced that the United States Food and Drug Administration has granted 510(k) clearance in the intervertebral disc space for Synthetic Bone Graft Putty (Amplify®). Amplify® represents a new class of synthetic biomaterials designed for the optimization of cell proliferation and bone formation and is comprised of Amplify® biphasic (HA/βTCP) ceramic granules suspended in an alkylene oxide polymer carrier. The device can be used either standalone or in combination with autograft bone (1:1 ratio) as a bone graft extender.
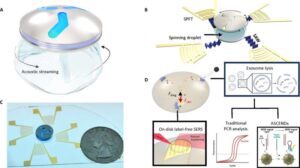
Announcing a new type of diagnostic platform that uses sound waves to spin an individual drop of water up to 6,000 revolutions per minute.

The collaborators aim to resolve the clinical, regulatory, coverage and payment questions facing developers of the devices.