
WAT Medical wins FDA nod for OTC migraine preventative device
HeadaTerm 2 uses neuromodulation technology. It releases targeted electrical impulses that increase the pain tolerance of the wearer.

HeadaTerm 2 uses neuromodulation technology. It releases targeted electrical impulses that increase the pain tolerance of the wearer.
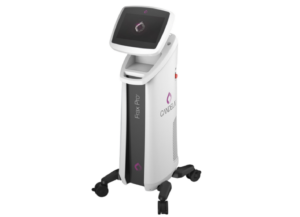
The Venus Versa Pro combines the applicator of the Venus Viva MD with the Venus Versa system which are both approved in Australia and registered in Australian Register of Therapeutic Goods (ARTG).

This milestone gives healthcare professionals an important tool for managing bacteremia by providing antibiotic susceptibility test (AST) results with unprecedented speed.

“This really is a landmark moment and we’ll be working with the NHS and others to ensure a fair rollout that reaches people as quickly as possible.”