
Neurovalens wins FDA nod for anxiety-treating neuromod device, raises $2.65M
Neurovalens announced today that it received FDA clearance for its Modius Stress device for treating anxiety and raised $2.65 million.

Neurovalens announced today that it received FDA clearance for its Modius Stress device for treating anxiety and raised $2.65 million.
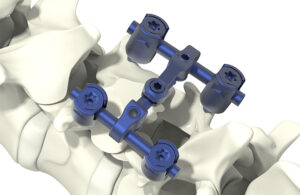
Orthobond has secured FDA de novo approval for its Ostaguard antibacterial technology that could one day be used for a wide range of medical devices — and beyond medtech.
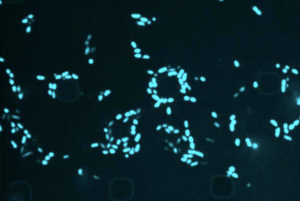
Most antibiotics target metabolically active bacteria, but with artificial intelligence, researchers can efficiently screen compounds that are lethal to dormant microbes.