Meditrina Announces CE Mark Approval and First Multi-International Cases Performed
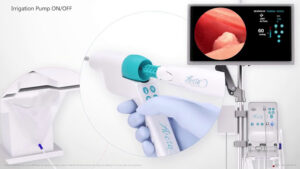
SAN JOSE, Calif., May 13, 2024 /PRNewswire/ — Meditrina, a leading innovator in gynecologic medical devices, announces the successful receipt of UKCA Mark, and CE Mark approval in accordance with Regulation (EU) 20147/745, for its state-of-the-art Aveta Hysteroscopy System. With this significant milestone achieved, Meditrina is thrilled to enter international markets, marking a pivotal moment in the company’s commitment to advancing women’s health globally.