
Dexcom launches Stelo over-the-counter CGM
Dexcom (Nasdaq: DXCM)+
today announced a significant product launch as another continuous glucose monitor (CGM) is hitting the market.

Dexcom (Nasdaq: DXCM)+
today announced a significant product launch as another continuous glucose monitor (CGM) is hitting the market.
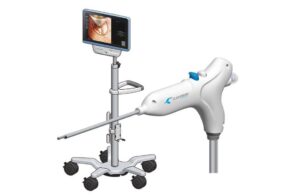
Clearmind Biomedical announced today that it received FDA 510(k) clearance for its Neuroblade neuroendoscopy system.
SUNRISE, Fla., Aug. 26, 2024 /PRNewswire/ — Obvius Robotics, a medical device company developing an innovative technology platform for democratizing vascular access, today announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its CERTA Access System for central venous catheterization (CVC).

The FDA clearance marks the first automated insulin delivery system for people with Type 2 diabetes.

WESTBOROUGH, Mass., Aug. 26, 2024 /PRNewswire/ — Olympus Corporation, a global medical technology company committed to making people’s lives healthier, safer, and more fulfilling, announced today the launch of two new jaw designs in the POWERSEAL™ Sealer/Divider family of advanced bipolar surgical energy products: the POWERSEAL Straight Jaw, Double-action (SJDA) and the POWERSEAL Curved Jaw, Single-action (CJSA). The first POWERSEAL device, launched in 2021, is the POWERSEAL Curved Jaw, Double-action (CJDA), which has established a strong foothold for Olympus in the advanced bipolar surgical energy market.