
AngioDynamics wins CE mark for atherectomy system
AngioDynamics (Nasdaq:ANGO) announced today that it received CE mark approval for its Auryon atherectomy system.

AngioDynamics (Nasdaq:ANGO) announced today that it received CE mark approval for its Auryon atherectomy system.

EYSINS, Switzerland, Sept. 3, 2024 /PRNewswire/ — At AliveDx, our mission is to empower diagnostic insights, transform patient care, and innovate for life. We are excited and proud to announce AliveDx has received IVDR-CE mark certification for its multiplexed MosaiQ AiPlex™ CD microarray immunoassay, designed to improve the accuracy and speed of diagnosing celiac disease
BOSTON and SHANGHAI, Sept. 3, 2024 /PRNewswire/ — Skyline Therapeutics, an innovation-driven gene therapy company committed to developing unique and novel solutions for rare and severe diseases, announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for SKG1108, a novel one-time intravitreally delivered gene therapy for the treatment of Retinitis Pigmentosa (RP).
PARIS, Sept. 3, 2024 /PRNewswire/ — European MedTech startup AZmed, recognized as a leading company in AI for medical imaging, today announced that its Rayvolve solution has received 510(k) clearance from the Food and Drug Administration (FDA) for detecting fractures on pediatric X-rays. This milestone comes two years after receiving FDA clearance for adult fracture detection, positioning Rayvolve as a top-performing solution for detecting fractures in adult and pediatric patients. The clearance was supported by an independent study conducted with SimonMed Imaging, one of the largest outpatient imaging providers in the U.S.

BELFAST, Northern Ireland, Sept. 3, 2024 /PRNewswire/ — B-Secur, a leader in advanced biosensing technology, proudly announces the FDA clearance and launch of HeartKey® Rhythm, a suite of electrocardiogram (ECG) algorithms and analytics designed to enhance clinical confidence, streamline industry efficiency, and improve outcomes for cardiac patients.
ENGLEWOOD, Colo., Sept. 3, 2024 /PRNewswire/ — Zynex Inc. (NASDAQ: ZYXI), a leading medical technology company specializing in non-invasive medical devices for pain management and rehabilitation, today announced FDA clearance of its new TensWave device
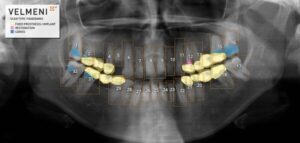
SUNNYVALE, Calif., Sept. 3, 2024 /PRNewswire/ — VELMENI announced today it received FDA 510(k) clearance for VELMENI for DENTISTS, an innovative AI that analyzes and annotates dental X-Rays

Embecta (Nasdaq:EMBC) today entered the insulin patch pump market with a major regulatory milestone.

Vitestro says it is the first company ever to achieve CE marking for an autonomous blood drawing device.
MIT engineers’ implantable ImPULS device could become an alternative to the electrodes now used to treat Parkinson’s and other diseases.