
FDA clears new stemless anatomical total shoulder implant from Smith+Nephew
Smith+Nephew (NYSE: SNN)+
announced that it received FDA clearance for its stemless anatomic total shoulder for the Aetos system.

Smith+Nephew (NYSE: SNN)+
announced that it received FDA clearance for its stemless anatomic total shoulder for the Aetos system.

CardioFocus announced today that investigators completed the first series of treatments in a study of its OptiShot pulsed-field ablation system.
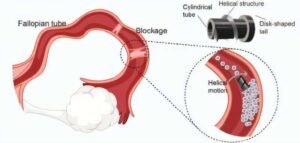
Infertility affects an estimated 186 million people worldwide, with fallopian tube obstruction contributing to 11–67% of female infertility cases. In AIP Advances researchers at the SIAT Magnetic Soft Microrobots Lab have developed an innovative solution using a magnetically driven robotic microscrew to treat fallopian tube blockages.
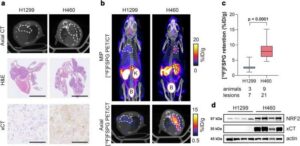
Researchers have used a chemical compound to light up treatment-resistant cancers on imaging scans, in a breakthrough that could help medical professionals better target and treat cancer.

ABBOTT PARK, Ill., Dec. 17, 2024 /PRNewswire/ — Abbott (NYSE: ABT) today announced the successful completion of the world’s first in-human leadless left bundle branch area pacing (LBBAP) procedures using the company’s investigational AVEIR™ Conduction System Pacing (CSP) leadless pacemaker system, as part of a feasibility study.