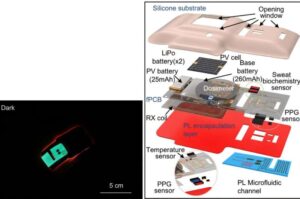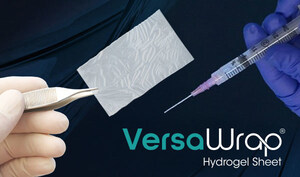
Alafair Biosciences Receives 510(k) Clearance for VersaCoat™ Tendon Protector and VersaCoat™ Nerve Protector
AUSTIN, Texas, July 30, 2025 /PRNewswire/ — Alafair Biosciences, a medical device company redefining soft-tissue protection in orthopedic surgery, announced today that it has received two 510(k) clearances from the FDA for VersaCoat™ Tendon Protector and VersaCoat™ Nerve Protector, marketed as a single product, VersaCoat™ Flowable Hydrogel.