Atraverse wins FDA clearance for left heart access device
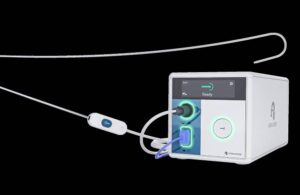
Atraverse Medical announced today that it received FDA clearance for its fully integrated Hotwire transseptal access system.

Atraverse Medical announced today that it received FDA clearance for its fully integrated Hotwire transseptal access system.

Boston Scientific (NYSE: BSX)+
announced recently that it received CE mark for its Farapoint pulsed field ablation (PFA) catheter.
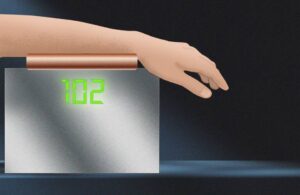
Researchers at MIT developed a non-invasive method for measuring blood glucose levels, potentially offering an alternative to fingersticks.
The trial will enroll patients who are undergoing nerve management procedures for severe neuropathic pain.

MED-EL’s cochlear implants are now FDA-approved in children seven months and older with bilateral sensorineural hearing loss (SNHL).

Partnership will expand Shoulder Innovations’ Disruptive Ecosystem with Advanced Enabling Technology, Complementing Surgeon and Patient Needs in the ASC

Three- and Four-Laser BD FACSDiscover A8 Cell Analysers Expand Accessibility of Spectral, Real-Time Imaging Cell Analysis

The approval is a major endorsement of Biomoneta’s scientific breakthrough and an important inflection point for Beyond Next Ventures India (BNV India)

Avance is an acellular nerve scaffold for the treatment of adult and pediatric patients aged 1 month or older with sensory, mixed, and motor peripheral nerve discontinuities

Today, NeuroVice announced that PATI is now commercially available, marking a breakthrough moment for millions of patients living with focal and grand-mal seizures.