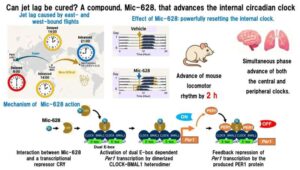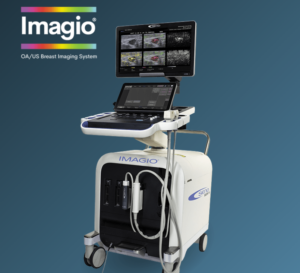
Seno Medical’s Next-Generation Imagio® Imaging System Obtains European Union (EU) Medical Device Regulation (MDR) CE Mark Certification
The CE Mark certification enables Seno Medical to market and sell the latest version of its Imagio® Imaging System with opto-acoustic, sound, and artificial intelligence in the European Union.