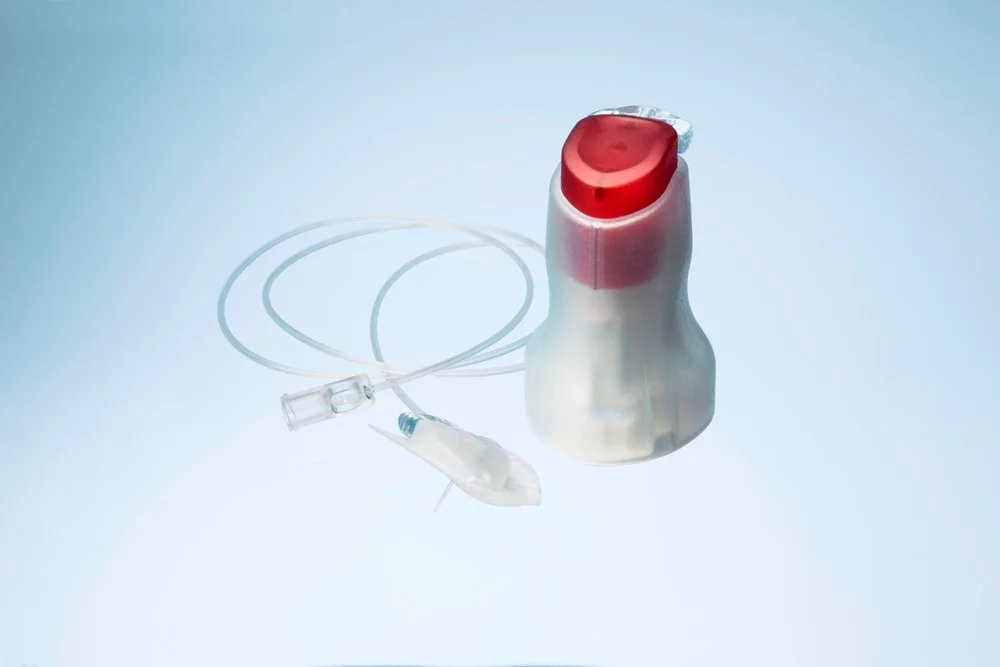
Convatec has achieved a key milestone with the U.S. FDA approval of subcutaneous apomorphine infusion for the treatment of advanced Parkinson’s disease, delivered via its Neria™ Guard infusion set. This innovative therapy offers a non-surgical, continuous drug delivery method that helps address the often-debilitating motor fluctuations experienced by Parkinson’s patients. Unlike traditional approaches that may involve invasive procedures, the system combines apomorphine hydrochloride with a small, portable infusion pump and a user-friendly insertion mechanism, offering a less burdensome and more accessible treatment path.
The Neria™ Guard infusion set is designed with safety, simplicity, and patient independence in mind. Its one-touch button technology minimizes human error and enhances ease of use, making it suitable for both home and clinical settings. Already widely used in Europe, the device has proven effective in supporting patients’ daily routines without interfering with quality of life. Its deployment in the U.S. market now offers healthcare providers and patients a much-needed alternative to existing Parkinson’s therapies, particularly for those who are not candidates for surgical interventions or who experience difficulties managing oral medications.