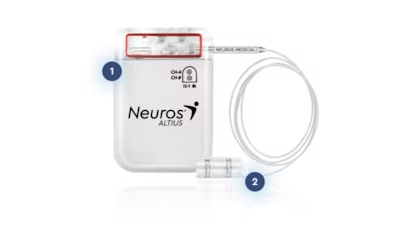
The Altius System is the only FDA-approved on-label device designed specifically for the treatment of Chronic Post-Amputation Pain for lower limb amputees. The patient- initiated, on-demand direct electrical nerve stimulation blocks painful signals from reaching the brain, thus providing durable pain relief without the potential dependency of opioid medications. The Altius System expands treatment options for lower limb amputees who have failed conventional therapies.
“The first commercial implantation of the Altius System represents a significant advancement in the treatment of a large unmet medical need in chronic post-amputation pain and a critical step forward for expanding new indications for neuromodulation therapies,” said David Veino, President and CEO of Neuros Medical. “We are proud to offer new hope to patients who have limited options for durable pain relief.”
The implant procedure was performed at Baylor Scott & White Heart and Vascular Hospital in Dallas, TX, one of several leading institutions participating in the early commercial launch of the Altius System.