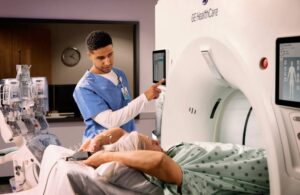
Chicago-based GE HealthCare designed the system to elevate the practice of coronary CT angiography (CCTA). It features unlimited one-beat cardiac imaging and integrated AI-powered tools. The company says it offers clinicians a fast, powerful and non-invasive way to identify potential heart blockages. It achieves this while enhancing patient comfort and streamlining workflow.
The system is engineered to address the most difficult cardiac exams, the company says. Those include patients with arrhythmias, heavily calcified coronaries and valves, stents or bypasses. The system’s unlimited one-beat cardiac imaging delivers full-heart clarity at low dose, even in cases without an ECG trace.
Features include ECG-less Cardiac, TrueFidelity DL, SnapShot Freeze 2 and Effortless Cardiac Workflow optimize image quality and reduce exam time, the company says. It helps to make cardiac CT more accessible and efficient.