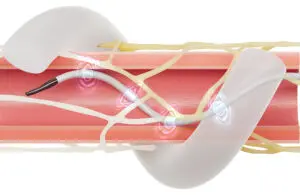
Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) granted approval for the blood pressure procedure for the treatment of resistant hypertension. Medtronic now plans to initiate the process to obtain approval for insurance coverage in the geography as well.
Approval in Japan means Symplicity Spyral is approved for commercial use in more than 75 countries.
This approval comes just a week after the company’s main RDN competition, Recor Medical, won approval in Japan for its Paradise platform. The two companies have been battling in the RDN space for years after Recor received a landmark FDA nod for the Paradise ultrasound RDN (uRDN) system in November 2023. Medtronic, the second company with such approval, picked up its nod for the Symplicity Spyral system just weeks later. The companies also have ongoing patent litigation related to their RDN systems.