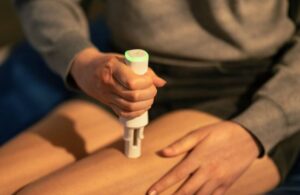
The device transforms the YpsoMate into a connected system that automatically captures and transmits injection data. Ypsomed said this marks an important step toward digital health solutions that offer deeper insight into therapy use. The digital add-on also enables objective monitoring and supports improved adherence and treatment outcomes.
Burgdorf, Switzerland-based Ypsomed said FDA clearance for SmartPilot demosntrates its readiness to support partners in clincial trials and commercial applications in the U.S.
SmartPilot captures key injection data like time and date, injection outcome and potential user errors. It transmits this data securely via Bluetooth to patients’ smartphones. Enabling automatic and real-time data collection supports both clinical development and day-to-day therapy management.