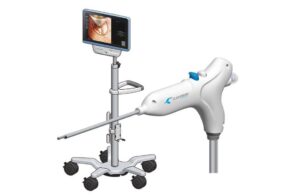
The novel system enables minimally invasive procedures with integrated features such as visualization, illumination, irrigation, suction, coagulation, and powered debridement. San Jose, California-based Clearmind also announced the first completed U.S. surgery using Neuroblade. Dr. Christopher Kellner performed the procedure at Mt. Sinai Hospital in New York.
Neuroblade features three components. First, it has a single-use multifunctional endoscope, called the Neuroblade. Next, it features Neuropad, a reusable, medical-grade tablet. Clearpath, the third component, is a disposable, transparent access sheath. Clearmind designed the system to improve the efficiency and outcomes of minimally invasive neurosurgical procedures.