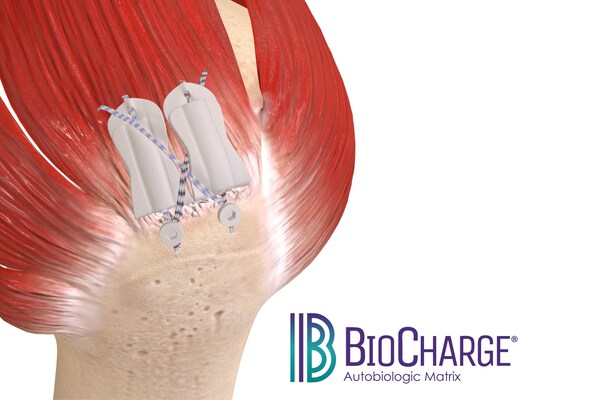
Expanding the Rotator Cuff Augmentation Portfolio
“We’re excited to expand our rotator cuff augmentation portfolio, building on the proven success of our flagship product, the ROTIUM® Bioresorbable Wick,” said Ronald Bracken, CEO of Atreon. “Retear rates remain high due to the complexities of tendon disease. With the introduction of BioCharge, Atreon is the only company offering solutions engineered to support native remodeling at both the tendon-bone and tendon-suture interfaces, resulting in improved treatment options for surgeons and their patients.”