MedTech News
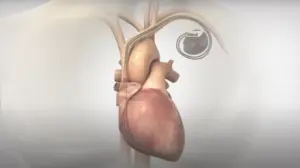
FDA approves expansion to Orchestra BioMed pacemaker trial
Orchestra BioMed (Nasdaq:OBIO) announced today that it began the rollout of a protocol update for its BACKBEAT study.

Nyxoah wins FDA nod for sleep apnea neuromod system
Nyxoah (Nasdaq:NYXH) announced today that it received FDA approval for its Genio neuromodulation device for treating sleep apnea.
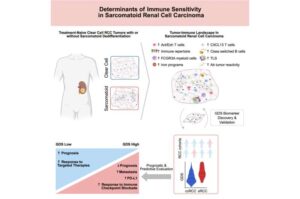
Genomics-guided tool helps guide immunotherapy choices for advanced kidney cancers
A study led by Roswell Park Comprehensive Cancer Center helps explain why a rare and hyper-aggressive subtype of kidney cancer is susceptible to immunotherapy—information that helped researchers create a first-of-its-kind tool to guide treatment decisions for advanced kidney cancers.

Mobile phone app reduces suicidal behavior among high-risk patients, new study shows
A mobile phone app designed to deliver suicide-specific therapy reduced suicidal behavior among high-risk psychiatric inpatients, according to a new study by scientists at Yale School of Medicine and The Ohio State University Wexner Medical Center and College of Medicine.
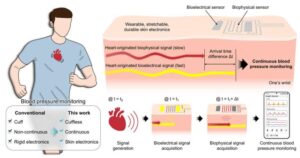
Wearable blood pressure monitor attaches like a bandage for real-time continuous measurement
Seoul National University College of Engineering announced that a research team has developed a wearable electronic device that attaches to the skin like a bandage and enables real-time, continuous monitoring of blood pressure over extended periods.

SurgiBox Achieves CE Mark for SurgiField System Devices
SurgiBox, a medical technology company committed to improving access to safe, clean surgery at the point of need, announced today it has received the CE Mark under MDR for the medical devices that comprise its flagship product, the SurgiField System.
Natural maple polyphenol found to inhibit tooth decay bacteria in new study
A new study in the journal Microbiology Spectrum highlights the potential of using a natural compound from maple to combat the bacteria responsible for tooth decay: Streptococcus mutans.
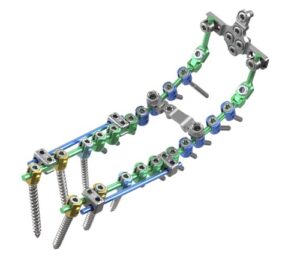
Full Market Launch of Cortium® Universal OCT Spinal Fixation System
PLANO, Texas, Aug. 7, 2025 /PRNewswire/ — ulrich medical USA, a leading medical device company focused on developing and commercializing spinal implant technologies, is proud to announce the full market launch of the Cortium® Universal OCT Spinal Fixation System, following a successful alpha release.
